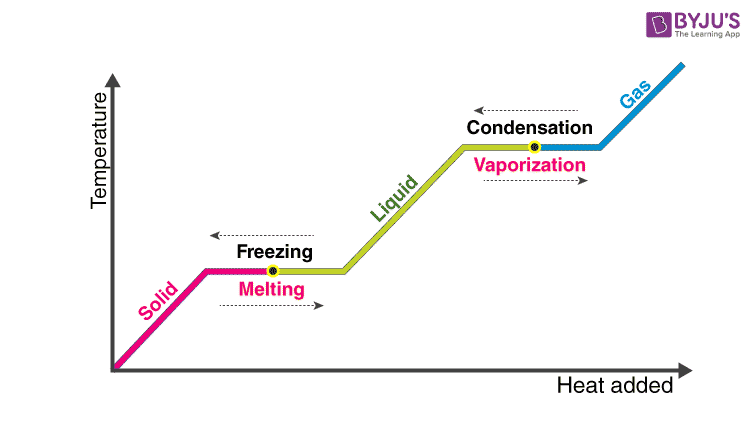
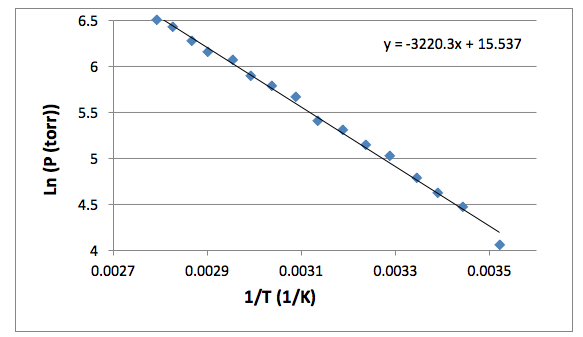
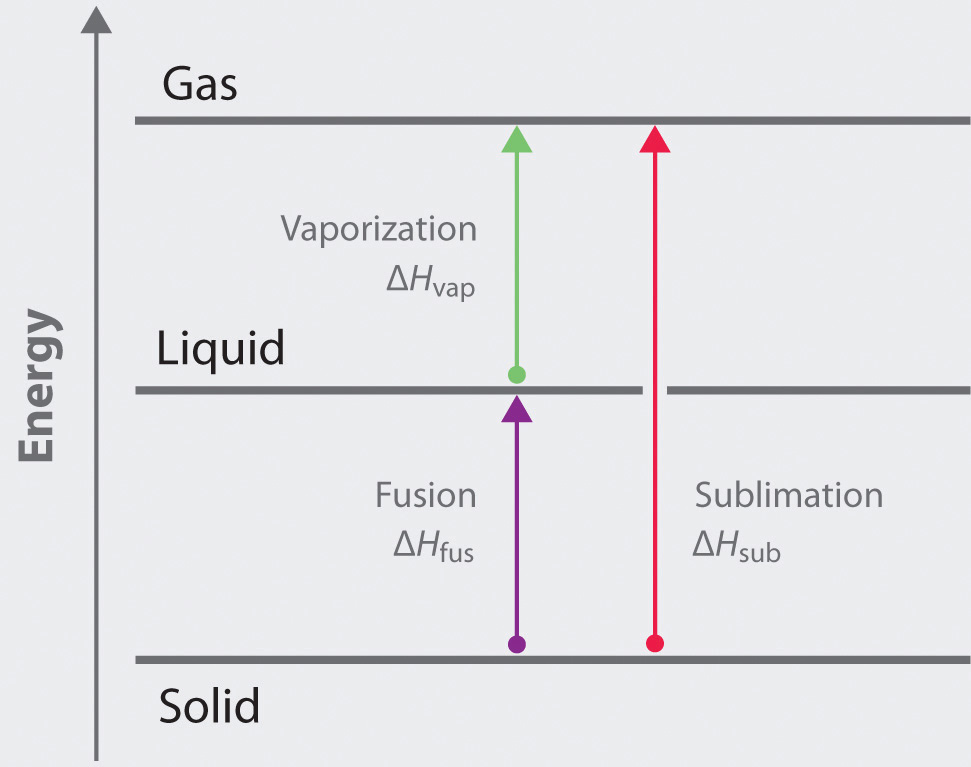
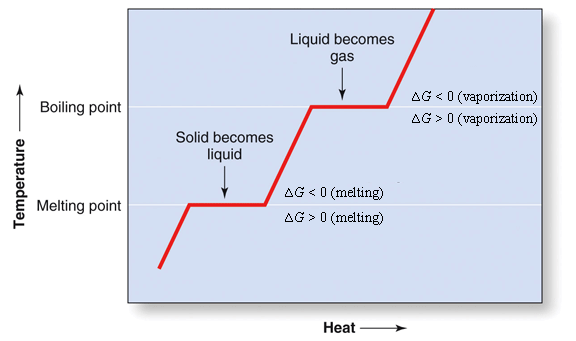
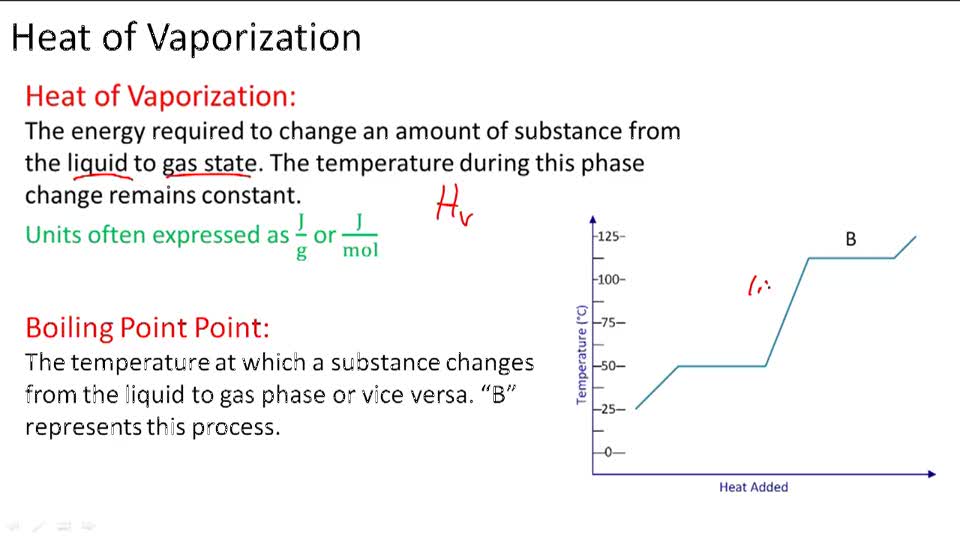
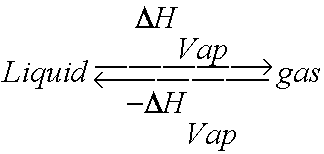11.3 Heat in Changes of State. Warm up Is it exo- or endo- thermic???? - negative ΔH -positive ΔH -Heat as a reactant -Heat as a product -Combustion of. - ppt download

Enthalpy of vaporization as a function of temperature–values obtained... | Download Scientific Diagram

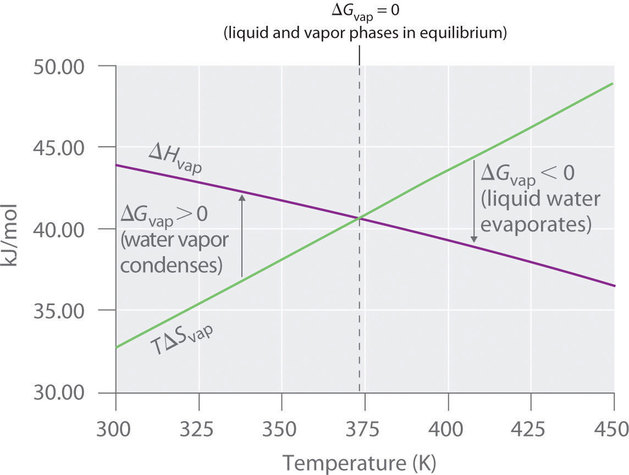
Describe (qualitatively) how standard enthalpy and entropy of vaporization of water will change with temperature. | Homework.Study.com

Enthalpy of vaporization of water: (—) Reference fundamental equation... | Download Scientific Diagram