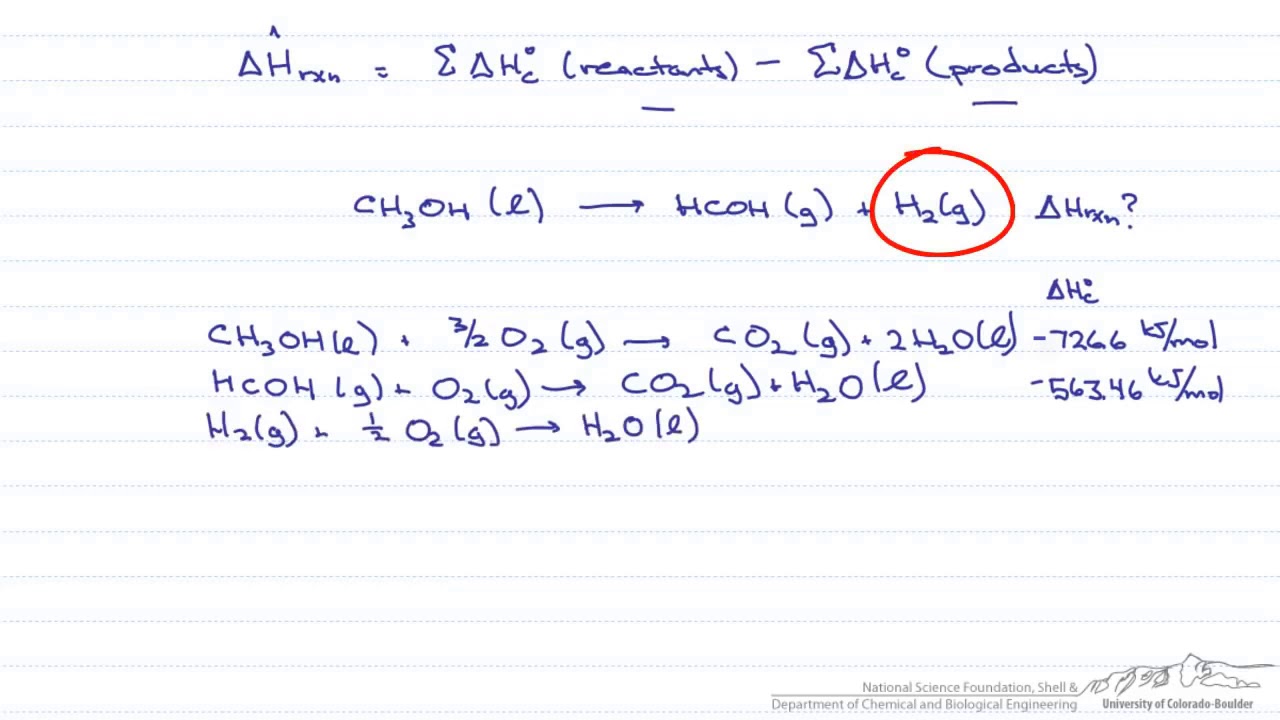Calculate the enthalpy of combustion of ethylene at 1 atm pressure and 298 K, if enthalpy of formation of CO2(g) , H2O(I) and C2H4(g) are - 394, - 242 and - 52 kJ respectively.

Calculate the heat of combustion of ethyl alcohol from the following data : (i) Heat of formation of ethyl alcohol = -64.1 kcal (ii) Heat of formation of water = -68.5 kcal (

Question Video: Calculating Standard Enthalpy of Combustion of Methane Using Standard Enthalpies of Formation of Methane and Carbon Dioxide | Nagwa

The heat of combustion of C(graphite) is - 393.5kJ mol^-1 . The heat of formation of CO2 from graphite is kJ mol^-1 .

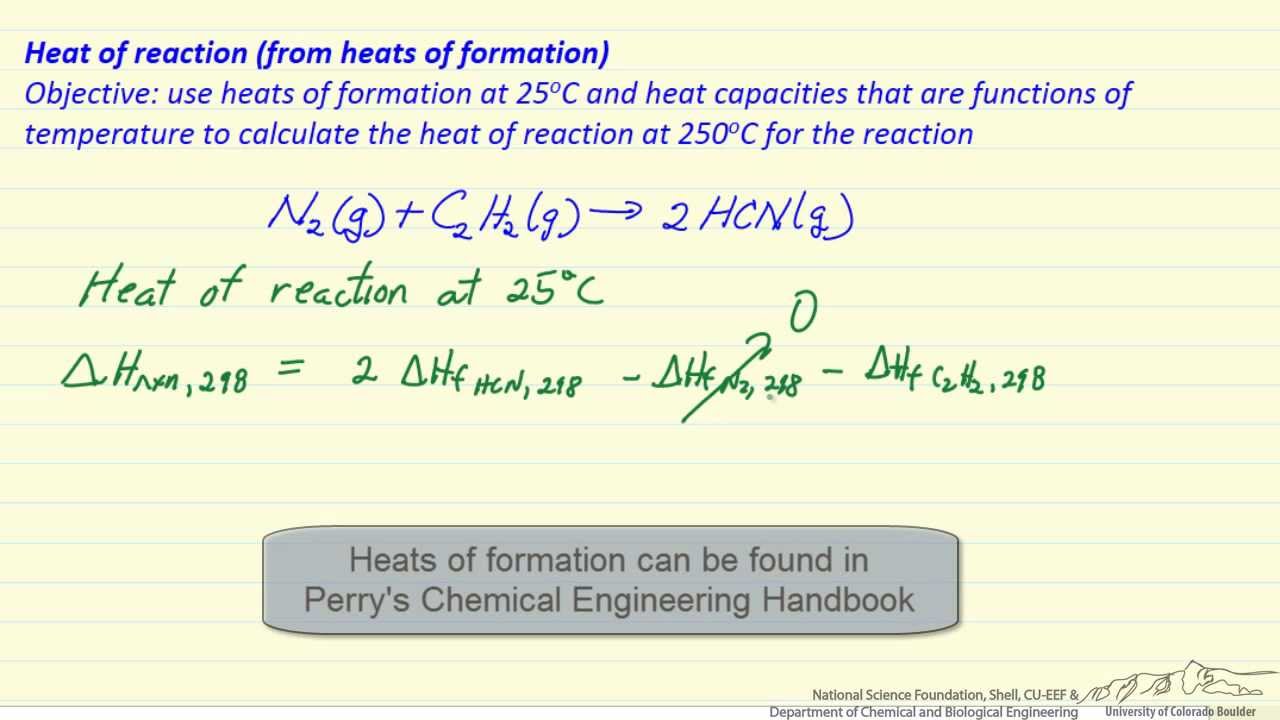
Question Video: Determining the Standard Enthalpy of Formation of Ethanol Using Standard Enthalpies of Combustion | Nagwa

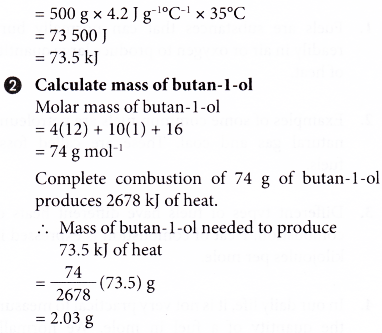
Molar Heat of Combustion Formula & Calculation | What is Heat of Combustion? - Video & Lesson Transcript | Study.com
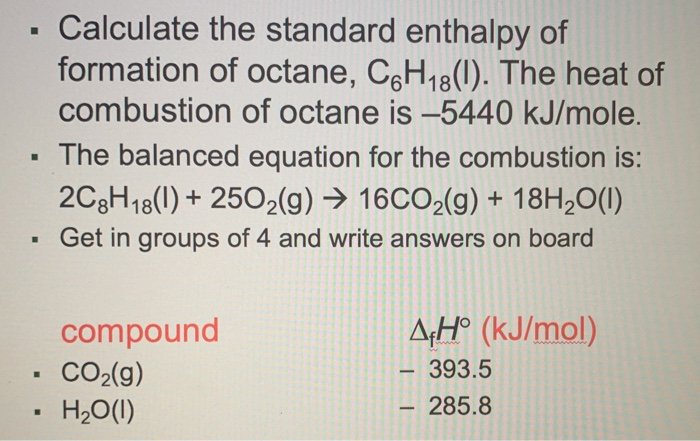
Calculate the standard heat of formation of propane, if its heat of combustion is -2220.2 KJ mol^-1 , the heats of formation - Sarthaks eConnect | Largest Online Education Community